Thermodynamics is a science in which we learn how thermal energy is flowing and transforming into other forms of energy. It also tells us whether this flow or transformation is feasible or not.
OR
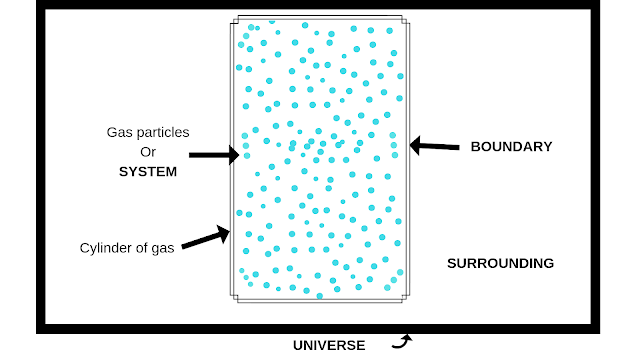
In thermodynamics, we learn about energy interacts between system and surrounding.Now you are wondering about the term system and surrounding. Let's learn them.
- System - A space or region which is under consideration for analysis or on which we do the analysis.
- Surrounding - Rest of the space or region other than the system.
Now there must be something which separate system and surrounding and that thing is the boundary.
- Boundary - As I already described a boundary is something that separates system and surrounding. It can be real or imaginary.
NOTE - The combination of system, surrounding and the boundary is called Universe.
# Types Of Boundary
- Rigid Boundary
- Movable Boundary
Rigid Boundary - It is a type of boundary which is fix for a system which is surrounded by that boundary.
Example - Boundary of a rigid cylinder.Movable Boundary - It is a type of boundary which is not fixed for a system which is surrounded by that boundary.
Example - Piston boundary of a piston-cylinder arrangement.
I hope these are easy ones and you are feeling confident but take a setup forward in learning more complicated things which I will make simple for you.😎
#Types Of Systems
- Open Systems
- Closed Systems
- Isolated Systems
Mass can go in and out, energy in any form like work and thermal can go in and out.
Example - engine(as they can take fuel and produce work which we used to run our vehicle), hairdryer.
Closed Systems - Those systems through which only energy transfer is possible no mass transfer is possible through these systems.
Example - Hot gas in a cylinder.
Isolated systems - Those systems which do not allow mass and energy transfer through them.
Example - Hot gas in an insulated cylinder, Thermos
I have a question for you which is given below-
Question - How you identify someone?
Answer - You identify someone by its characteristics, like height, weight, sex, colour etc.
In the same way, the system has some characteristics like smell, colour, pressure, volume, density etc. Here we are focusing on the system's smell, colour rather on pressure, volume, density etc i.e. we are focusing on characteristics which are measurable.
#Properties
These are the characteristics which we can measure like density, volume, mass, Temperature etc.
All properties are categorized in the following category.
- Intensive Properties
- Extensive Properties
Intensive Properties - Those properties which do not vary with the mass of the systems at a defined state of a system. Example - density, temperature, pressure etc.
Extensive Properties - Those properties which vary with the mass of the system at a defined state of the system.
Example - All type of energies, mass, volume etc.
NOTE - In the above definitions you have encountered a term 'state'.
#State - In simple words, it represents the condition of a system.
#Process - A change in the state of the system leads a process.
#Path - It's the way through which a system undergoes a process.
#Internal Energy - It's the energy associated with all the molecules of a system like Bonding energy( energy present in the bond of two elements), Lattice vibration etc.
#Point Function and Path Function
1. Point Functions - Those functions which are independent of the path are called Path Functions. All properties are Point Functions as these properties are defined for a state.
These are exact differentials because they change in these function during processes does not vary with the path.
Example - Pressure, Volume, all energies etc.
2. Path Functions - Those functions which depend on the path which a process follow during the change of state. These are inexact differentials because they vary with the variation in the path.
Example - Work, Heat, Entropy Generation.
So, this is all off for this post at and I hope you like this post and if you have any query for the above content please comment it.👇🏻
In the end, I wanna say Stay Hungary Stay Foolish and I will see you in the next Post.
🤘🏻🤘🏻🤘🏻🤘🏻



Comments
Post a Comment